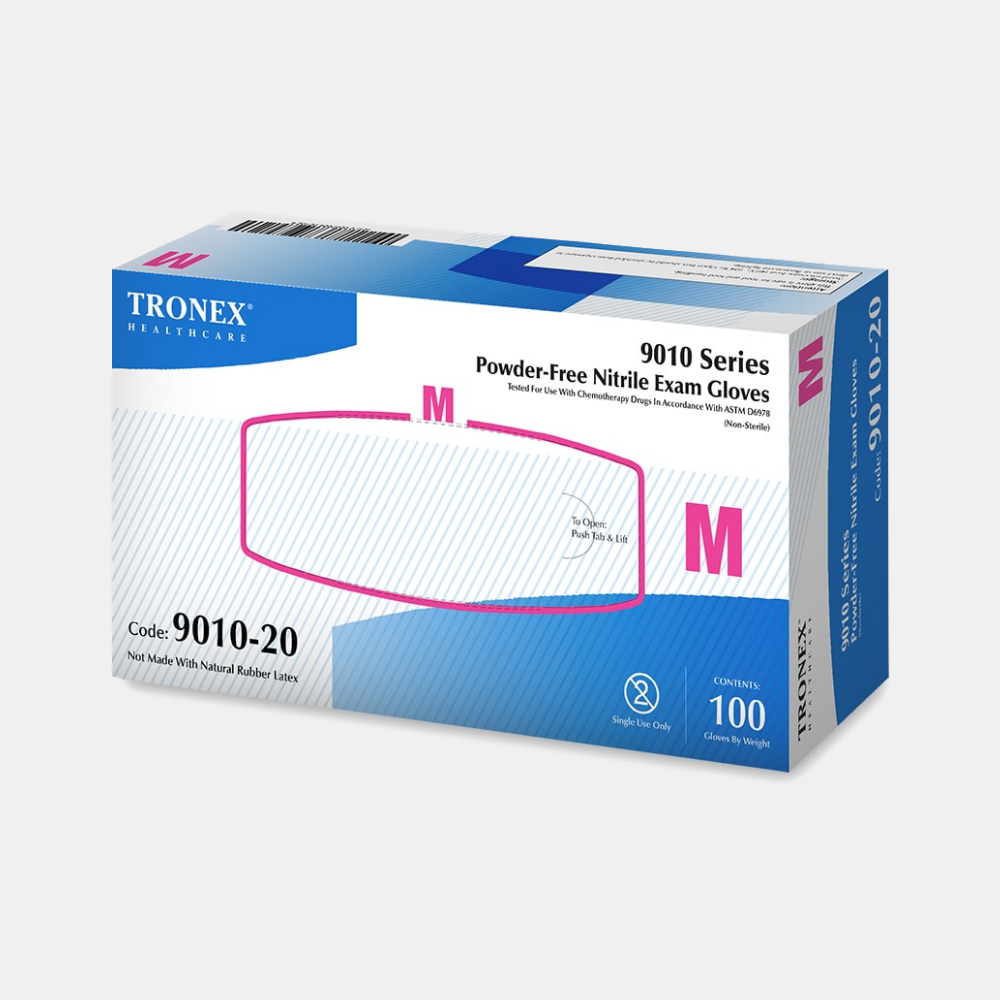
Description
Exclusive nitrile material formulation with added thickness features a fully textured surface for enhanced durability and abrasion resistance. Provides excellent barrier protection from bloodborne pathogens, as well as exceptional resistance to chemotherapy drugs and other chemicals.
* FDA 510(k) Approved as a Medical Device.
* Meets and exceeds ASTM D6319 Specification for Nitrile Examination Gloves for Medical Application.
* Meets and Exceeds ASTM F1671 Test Method for Resistance of Materials by Blood-Borne Pathogens (Viral Penetration).
* Meets and exceeds ASTM D6978 Resistance of Medical Gloves to Permeation by Chemotherapy Drugs.
* Manufacturing certified ISO 9001 for Quality Management Systems, as well as ISO 13485 for Quality Management Systems for Medical Devices.






Colin J. –
This glove is perfect for fixing cars. Because it is oil resistance. And it fit my hands perfectly, help me perform my jobs easily
Amy L. –
love it
Diana E. –
The quality is actually very good
Mile N. –
my mom and mom in law all like it
Gina Q. –
good
Dolly T. –
good quality
Elizabeth D. –
I read this article online a few months ago about how many germs our hands carry. It made me think. As a full-time mom, I take care of my babies and family at a daily basis. I can’t imagine how many germs transformed from my hands to their food. So I decided to try this one. It has no powder, no smell. I can handle salads after I handle the steak. It just makes my life much much easier.
Jack X. –
quality gloves
Robert A. –
Great quality
Gary W. –
Very stretchy. I love them
Jennifer K. –
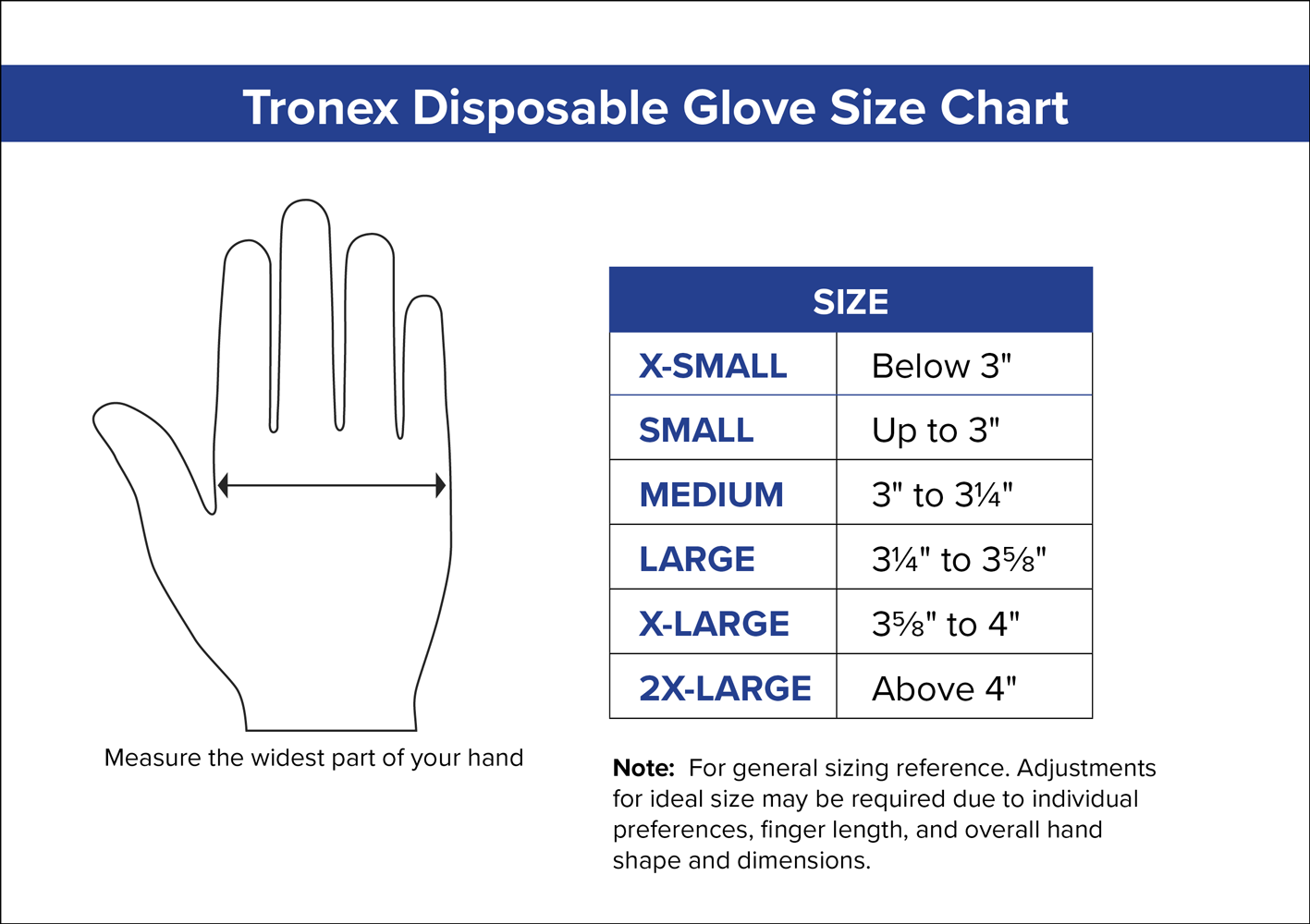
I like this one. It meets my needs at work in the grocery store. Maybe I should get one size bigger next time. I would need to compare the two sizes.
Tammy K. –
good
Stephan C. –
Tronex 9010 is the best chemo glove I have ever used so far. Durable and fits well.
Mark Horning –
I found the website very easy to navigate and quickly find the items. Delivery was great. I recommend North Starlight for all. your hygiene products.